Gel-Strength-Test-System
0 likes | 19 Views
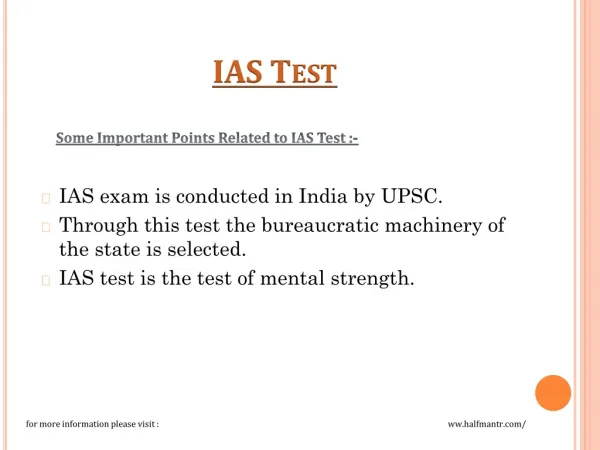
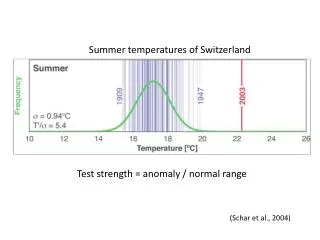
Gel Strength Test System is designed primarily for the precise measurement of mechanical properties like gel strength in gelatine within a controlled environmental condition. It further enhances flexibility by allowing users to adjust the sampling depth within a range of 1 to 60 mm. This system offers remarkable accuracy with a travel accuracy of u00b10.3% and a generous test range spanning from 5 to 1000 g Bloom.<br>It finds applications in mechanical transmission gear systems with shock absorbers, and refrigerators, offering high-speed operation with minimal noise. It is employed in the food, pharm
Download Presentation 

Gel-Strength-Test-System
An Image/Link below is provided (as is) to download presentation
Download Policy: Content on the Website is provided to you AS IS for your information and personal use and may not be sold / licensed / shared on other websites without getting consent from its author.
Content is provided to you AS IS for your information and personal use only.
Download presentation by click this link.
While downloading, if for some reason you are not able to download a presentation, the publisher may have deleted the file from their server.
During download, if you can't get a presentation, the file might be deleted by the publisher.
E N D
Presentation Transcript
More Related